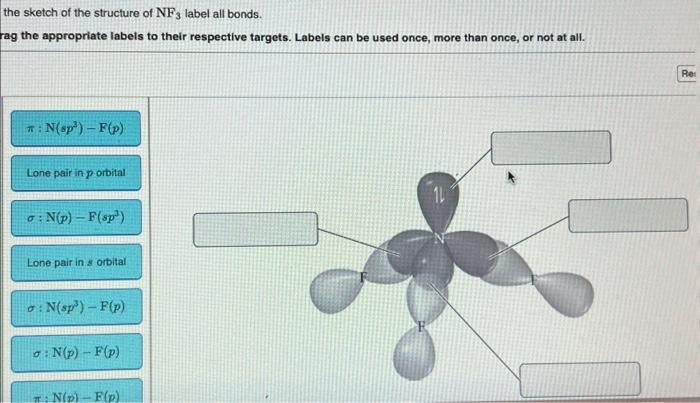
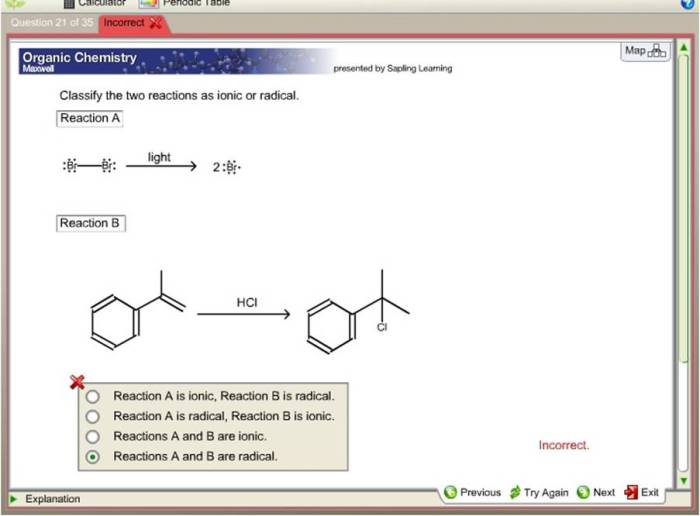
Classify the two reactions as ionic or radical – Ionic and radical reactions are two fundamental types of chemical reactions that exhibit distinct characteristics and mechanisms. This article aims to provide a comprehensive overview of these reactions, enabling readers to identify, understand, and differentiate between them.
Ionic and Radical Reactions
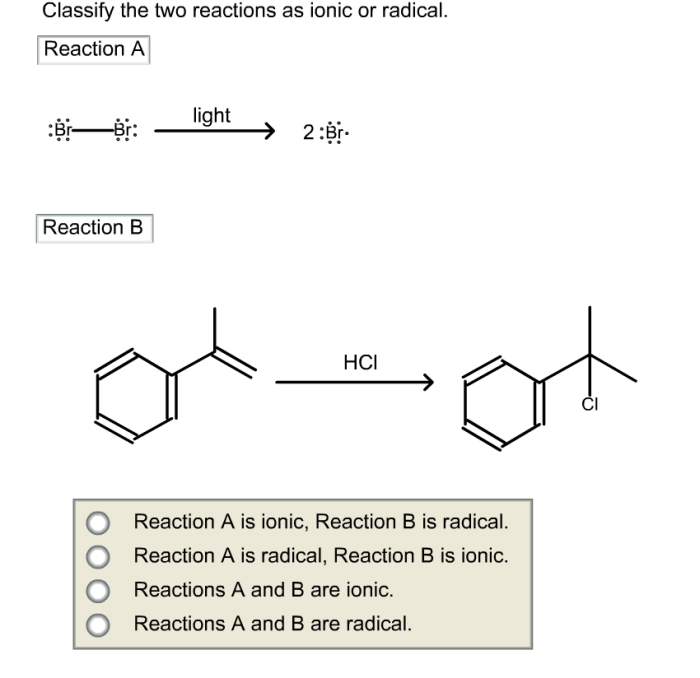
Ionic reactions involve the transfer of electrons between atoms or ions, resulting in the formation of ions. In contrast, radical reactions involve the formation and reactions of free radicals, which are species with unpaired electrons.
Key Differences between Ionic and Radical Reactions
- Mechanism:Ionic reactions occur through the transfer of electrons, while radical reactions involve the formation and reactions of free radicals.
- Polarity:Ionic reactions typically occur in polar solvents, while radical reactions can occur in both polar and nonpolar solvents.
- Rate:Ionic reactions are generally faster than radical reactions.
- Selectivity:Ionic reactions are more selective than radical reactions.
Identifying Ionic and Radical Reactions
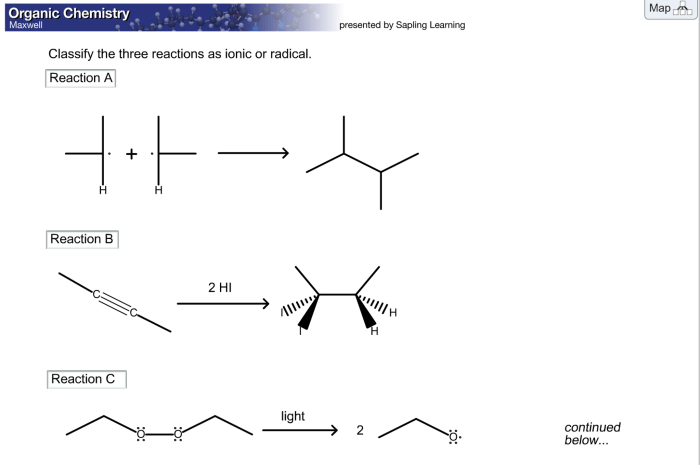
| Reaction Type | Example | Characteristics |
|---|---|---|
| Ionic | NaCl + AgNO3 → AgCl + NaNO3 | – Transfer of electrons
|
| Radical | CH4+ Cl 2→ CH 3Cl + HCl | – Formation of free radicals
|
Mechanisms of Ionic and Radical Reactions
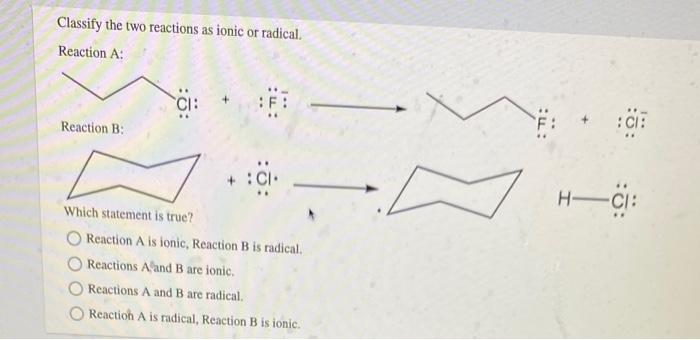
Ionic Reactions
Ionic reactions proceed through the transfer of electrons between atoms or ions. The transfer of electrons creates oppositely charged ions, which are attracted to each other by electrostatic forces.
Radical Reactions, Classify the two reactions as ionic or radical
Radical reactions involve the formation and reactions of free radicals. Free radicals are species with unpaired electrons, which makes them highly reactive. Radical reactions can occur through various mechanisms, including homolytic bond cleavage, heterolytic bond cleavage, and radical recombination.
Applications of Ionic and Radical Reactions: Classify The Two Reactions As Ionic Or Radical
Ionic Reactions
- Electroplating
- Battery reactions
- Production of salts
Radical Reactions, Classify the two reactions as ionic or radical
- Polymerization reactions
- Combustion
- Biological processes (e.g., metabolism)
Q&A
What are the key differences between ionic and radical reactions?
Ionic reactions involve the transfer of electrons between atoms or ions, while radical reactions involve the formation and propagation of free radicals.
How can we identify ionic and radical reactions?
Ionic reactions typically occur in polar solvents and involve the formation of ions, while radical reactions often occur in nonpolar solvents and involve the formation of free radicals.
What are some applications of ionic and radical reactions?
Ionic reactions are used in a wide range of applications, including electroplating, batteries, and the production of many inorganic compounds. Radical reactions are used in a variety of applications, including polymerization, combustion, and the production of many organic compounds.