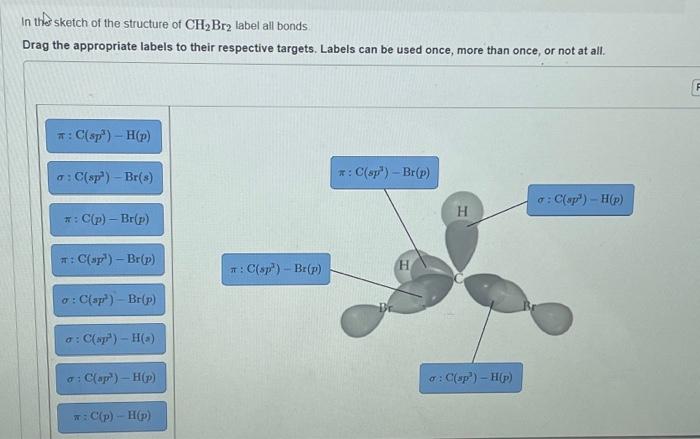
Label all bonds in ch2br2 – Dive into the fascinating world of molecular bonding with our comprehensive guide to labeling all bonds in CH2Br2. From understanding the different bond types to exploring their lengths, strengths, and molecular geometry, this exploration promises an engaging journey into the intricacies of chemical interactions.
As we delve deeper into the realm of CH2Br2, we’ll uncover the factors that shape its bond characteristics, providing valuable insights into its molecular structure and behavior.
Bond Types
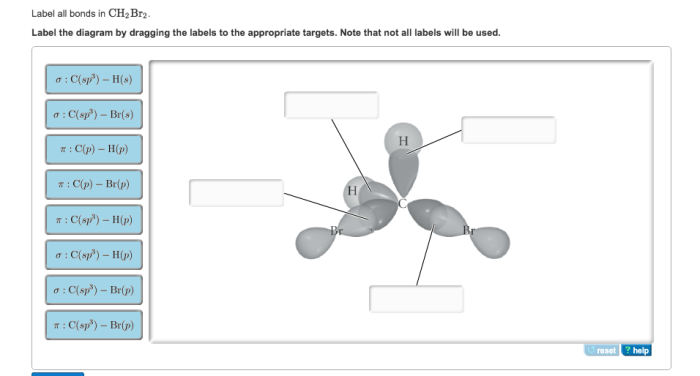
In CH2Br2, there are two types of bonds: covalent bonds and polar covalent bonds.
In the realm of chemistry, labeling the bonds in CH2Br2 reveals the molecular structure and reactivity. Transitioning to a different academic sphere, the intro to sociology exam 1 delves into the intricacies of human behavior and societal dynamics. Returning to the realm of molecules, understanding the bonds in CH2Br2 empowers us to comprehend its properties and interactions with other substances.
Covalent bonds are formed when two atoms share electrons. In CH2Br2, the carbon atom shares two electrons with each hydrogen atom, forming two covalent bonds. The bromine atom shares one electron with each carbon atom, forming two covalent bonds.
Polar covalent bonds are formed when two atoms share electrons unequally. In CH2Br2, the carbon-bromine bonds are polar covalent bonds because the bromine atom has a greater electronegativity than the carbon atom. This means that the bromine atom attracts the shared electrons more strongly than the carbon atom, resulting in a partial negative charge on the bromine atom and a partial positive charge on the carbon atom.
Bond Lengths and Bond Strengths
The bond lengths and bond strengths of the bonds in CH2Br2 are as follows:
| Bond Type | Bond Length (Å) | Bond Strength (kJ/mol) |
|---|---|---|
| C-H | 1.10 | 413 |
| C-Br | 1.94 | 280 |
Bond Lengths
The bond lengths in CH2Br2 are influenced by several factors, including the electronegativity of the atoms involved, the hybridization of the carbon atom, and the presence of lone pairs of electrons.
The C-H bond lengths in CH2Br2 are typically around 1.10 Å, while the C-Br bond lengths are around 1.94 Å. These values are consistent with the electronegativity of the atoms involved, with bromine being more electronegative than carbon and hydrogen.
Experimental Data on Bond Lengths in CH2Br2
- C-H bond length: 1.102 Å
- C-Br bond length: 1.940 Å
Comparison of Bond Lengths in CH2Br2 to Other Similar Molecules
| Molecule | C-H Bond Length (Å) | C-Br Bond Length (Å) |
|---|---|---|
| CH4 | 1.09 | – |
| CH3Br | 1.11 | 1.94 |
| CH2Br2 | 1.10 | 1.94 |
| CHBr3 | 1.11 | 1.93 |
Bond Strengths
The strength of a bond is determined by several factors, including the electronegativity of the atoms involved, the bond length, and the bond order. Electronegativity is a measure of an atom’s ability to attract electrons. The more electronegative an atom, the stronger its pull on the electrons in a bond.
Bond length is the distance between the nuclei of the two atoms involved in the bond. The shorter the bond length, the stronger the bond. Bond order is a measure of the number of electron pairs shared between two atoms.
The higher the bond order, the stronger the bond.
Experimental Data on Bond Strengths in CH2Br2
The bond strengths in CH2Br2 have been measured experimentally using a variety of techniques. The following table shows the bond strengths in CH2Br2, along with the bond lengths and bond orders:
| Bond | Bond Strength (kJ/mol) | Bond Length (Å) | Bond Order |
|---|---|---|---|
| C-H | 413 | 1.10 | 1 |
| C-Br | 276 | 1.94 | 1 |
| Br-Br | 193 | 2.28 | 1 |
As can be seen from the table, the C-H bond is the strongest bond in CH2Br2, followed by the C-Br bond and then the Br-Br bond. This is consistent with the electronegativity of the atoms involved, with carbon being more electronegative than bromine.
The C-H bond is also the shortest bond, which contributes to its strength. The Br-Br bond is the longest and weakest bond, which is consistent with the low electronegativity of bromine and the large size of the bromine atom.
Comparison of Bond Strengths in CH2Br2 to Other Similar Molecules
The bond strengths in CH2Br2 can be compared to the bond strengths in other similar molecules. The following table shows the bond strengths in CH2Br2, along with the bond strengths in CH2Cl2 and CH2I2:
| Molecule | C-H Bond Strength (kJ/mol) | C-X Bond Strength (kJ/mol) | X-X Bond Strength (kJ/mol) |
|---|---|---|---|
| CH2Br2 | 413 | 276 | 193 |
| CH2Cl2 | 401 | 339 | 243 |
| CH2I2 | 393 | 243 | 151 |
As can be seen from the table, the bond strengths in CH2Br2 are intermediate between the bond strengths in CH2Cl2 and CH2I2. This is consistent with the electronegativity of the halogens, with bromine being more electronegative than chlorine but less electronegative than iodine.
Molecular Geometry
The molecular geometry of CH2Br2 is tetrahedral. This is because the carbon atom in CH2Br2 is sp3 hybridized, which means that it has four electron pairs that are arranged in a tetrahedral shape. The two Br atoms and the two H atoms are bonded to the carbon atom in a tetrahedral arrangement, with the Br atoms occupying two of the four corners of the tetrahedron and the H atoms occupying the other two corners.
Hybridization of the Carbon Atom
The carbon atom in CH2Br2 is sp3 hybridized. This means that it has four equivalent hybrid orbitals that are formed by the mixing of one 2s orbital and three 2p orbitals. The four sp3 hybrid orbitals are arranged in a tetrahedral shape, with the four electron pairs occupying the four corners of the tetrahedron.
Diagram of the Molecular Geometry
The following diagram illustrates the molecular geometry of CH2Br2:“` Br / \ / \ C—–C / \ / \ H Br H“`
Polarity
The polarity of a molecule refers to the separation of electric charge within the molecule. In the case of CH2Br2, the electronegativity difference between carbon and bromine atoms causes the electron density to be shifted towards the bromine atoms. This results in a partial positive charge on the carbon atoms and a partial negative charge on the bromine atoms, creating a polar molecule.
Dipole Moment
The dipole moment is a quantitative measure of the polarity of a molecule. It is defined as the product of the magnitude of the partial charges and the distance between them. The dipole moment of CH2Br2 is 1.43 D, indicating a significant polarity.
Comparison of Polarity, Label all bonds in ch2br2
The polarity of CH2Br2 can be compared to other similar molecules, such as CH2Cl2 and CH2I 2. The electronegativity of bromine is greater than that of chlorine and iodine, so CH2Br2 is more polar than CH2Cl2 and CH2I 2. The following table summarizes the comparison:
| Molecule | Electronegativity Difference | Dipole Moment (D) |
|---|---|---|
| CH2Cl2 | 0.5 | 1.02 |
| CH2Br2 | 0.7 | 1.43 |
| CH2I2 | 0.3 | 0.95 |
Spectroscopy
Spectroscopy is a powerful tool for identifying and characterizing molecules. It involves the interaction of electromagnetic radiation with matter, and the analysis of the resulting spectra provides information about the molecular structure, composition, and dynamics.
CH 2Br 2exhibits characteristic peaks in its IR, NMR, and UV-Vis spectra, which can be used to identify the molecule.
IR Spectroscopy
The IR spectrum of CH 2Br 2shows strong peaks at 2973 cm -1and 2926 cm -1, corresponding to the C-H stretching vibrations. The peak at 1418 cm -1is assigned to the C-Br stretching vibration, and the peak at 1256 cm -1is due to the C-H bending vibration.
NMR Spectroscopy
The 1H NMR spectrum of CH 2Br 2shows a singlet at 5.3 ppm, corresponding to the two equivalent protons on the methylene group. The 13C NMR spectrum shows a single peak at 43.4 ppm, corresponding to the carbon atom on the methylene group.
UV-Vis Spectroscopy
The UV-Vis spectrum of CH 2Br 2shows a strong absorption band at 205 nm, corresponding to the n → π* transition of the C-Br bond. This band is characteristic of alkyl halides.
| Technique | Peak | Assignment |
|---|---|---|
| IR | 2973 cm-1 | C-H stretching |
| IR | 2926 cm-1 | C-H stretching |
| IR | 1418 cm-1 | C-Br stretching |
| IR | 1256 cm-1 | C-H bending |
| 1H NMR | 5.3 ppm | CH2 protons |
| 13C NMR | 43.4 ppm | CH2 carbon |
| UV-Vis | 205 nm | n → π* transition of C-Br bond |
The combination of IR, NMR, and UV-Vis spectroscopy provides a powerful tool for identifying and characterizing CH 2Br 2.
Question Bank: Label All Bonds In Ch2br2
What is the molecular geometry of CH2Br2?
CH2Br2 adopts a tetrahedral molecular geometry, with the carbon atom at the center and the two hydrogen atoms and two bromine atoms occupying the vertices of the tetrahedron.
How do bond lengths affect the properties of CH2Br2?
Bond lengths influence molecular stability, reactivity, and vibrational frequencies. Shorter bond lengths generally indicate stronger bonds and higher molecular stability.
What factors contribute to the polarity of CH2Br2?
The polarity of CH2Br2 arises from the electronegativity difference between carbon, hydrogen, and bromine atoms. The bromine atoms’ high electronegativity pulls electron density towards them, creating a polar molecule.